Answer:
D. 8; 0.15 g
Explanation:
We have been given that a a laboratory has a 38 g sample of polonium-210. The half-life of polonium-210 is about 138 days.
To find the half-lives of polonium-210, we will divide 1104 by 138.
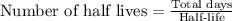
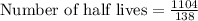
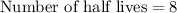
Therefore, there are 8 half-lives will occur in 1104 days.
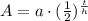
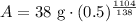
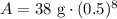
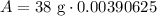
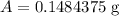
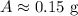
Therefore, 0.15 gm of the polonium will be left after 1104 days.