Answer: The major ions present in aqueous LiOH solution is lithium ions and hydroxide ions.
Step-by-step explanation:
Ionization reaction is defined as the reaction in which an ionic compound dissociates into its ions when dissolved in aqueous solution.
The chemical equation for the ionization of LiOH solution follows:
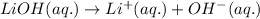
By Stoichiometry of the reaction:
1 mole of lithium hydroxide produces 1 mole of lithium ions and 1 mole of hydroxide ions
Hence, the major ions present in aqueous LiOH solution is lithium ions and hydroxide ions.