Na (sodium) has a molar mass of 23g.
Cl (chlorine) has a molar mass of 35g.
To find the molar mass of NaCl, add the individual elements' masses together:
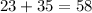
The molar mass of NaCl is 58g.
To find the mass of 3.45 moles of NaCl, multiply the moles by its mass:
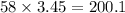
There are
200.1 grams in 3.45 moles of NaCl.