we can use the combined gas law equation for this question.
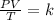
where P - pressure
V - volume
T - temperature in kelvin
and k is constant
therefore
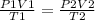
parameters for the STP conditions are on the left side and parameters for the given instance
T1 - standard temperature - 273 K
T2 - 18 °C + 273 = 291 K
P1 - standard pressure - 1 atm
substituting the values in the equation
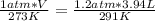
V = 4.4 L
new volume STP is 4.4 L