Answer: non polar covalent
Step-by-step explanation:
1. An ionic bond is formed when an element completely transfers its valence electron to another element.
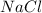
2. A covalent bond is formed when an element shares its valence electron with another element. This bond is formed between two non metals.
A covalent bond is of two types :
a) A polar covalent bond is defined as the bond which is formed when there is a difference of electronegativities between the atoms. The more electronegative element withdraws the electron density towards itself , thus generating a partial negative charge and the lesser electronegative element bears a partial positive charge.Example:
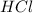
b) A non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms. Thus both atoms exert the same pull on shared electrons. Example:
