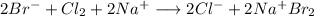Answer:
The products are sodium chloride (
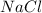 ) and bromine (
) and bromine (
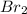 )
)
Step-by-step explanation:
Hi, when the chlorine gas is bubbled it produces a redox reaction because of its high reactivity with the sodium compared to the bromine.
So you will have to hemireactions:

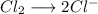
Combining both equations and adding the sodium: