Answer: The distribution of electrons around helium atom is given in the image attached below.
Step-by-step explanation:
Helium is the 2nd element of the periodic table having electronic configuration of
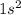
The electrons are arranged in orbits around the atom.
In the given element, 2 electrons are arranged in the lowest energy orbit, which is '1s'
Hence, the distribution of electrons in helium atom is shown below.