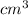The new volume of balloon when the temperature increases to 47 degree C is 209
Step-by-step explanation:
According to temperature-volume law, It states that the volume of a gas is directly proportional to the Kelvin temperature, while pressure and amount are held constant.
The equation is
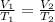
Where V is volume and T is temperature in Kelvins. We know that
V1=
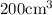
T1=
 +273=306 K
+273=306 K
T2=
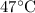 +273=320 K
+273=320 K
To find V2, after Rearranging.
V2=
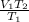
V2 =
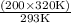 = 209
= 209