Answer: Option (D) is the correct answer.
Step-by-step explanation:
A chemical reaction is defined as the reaction which causes change in chemical composition of a substance.
According to law of conservation of mass, mass can neither be created nor it can be destroyed. It can only be changed from one form to another.
For example,
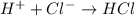
Here, total mass of reactants = (1.008 + 35.453) g/mol = 36.461 g/mol
Whereas total mass of products = 36.461 g/mol
This shows that mass remains the same before or after the reaction.
Similarly, the number of atoms of the type involved will remains the same as no change in their nucleus is occurring.
Thus, we can conclude that the following must be the same before and after a chemical reaction.
- The sum of the masses of all substances involved.
- The number of atoms of the type involved.