Answer: It is true that the given example is an endothermic reaction.
Step-by-step explanation:
Endothermic reaction is defined as the reaction in which reactant species absorb heat from the surrounding or from a source.
For example,
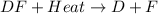 is an endothermic reaction.
is an endothermic reaction.
Energy of products is more than the energy of reactants in an endothermic reaction.
On the other hand, a reaction in which heat is released by reactant species is known as an exothermic reaction.
For example,
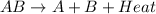 is an exothermic reaction.
is an exothermic reaction.
Energy of products is less than the energy of reactants in an exothermic reaction.
Hence, we can conclude that the given equation,
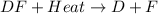 , is an example of an endothermic reaction.
, is an example of an endothermic reaction.