Answer: Option (b) is the correct answer.
Step-by-step explanation:
A hydrocarbon chain that contains a functional group "-OH" is known as an alcohol.
When "-OH" group is attached at the first carbon of a hydrocarbon then it is known as primary alcohol. For example,
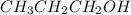 is propanol and it is a primary alcohol.
is propanol and it is a primary alcohol.
When "-OH" group is attached at the second carbon of a hydrocarbon then it is known as primary alcohol. For example,
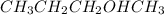 is 2-butanol and it is a secondary alcohol.
is 2-butanol and it is a secondary alcohol.
When "-OH" group is attached at the central carbon of a hydrocarbon then it is known as tertiary alcohol. For example,
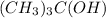 is 1,1-Dimethylethanol and it is a tertiary alcohol.
is 1,1-Dimethylethanol and it is a tertiary alcohol.
Therefore, we can conclude that a tertiary has a -OH radical bonded to a central carbon atom.