Answer: There are 6 hydrogen atoms present in 2 moles of hydroxylamine.
Step-by-step explanation:
Hydroxylamine has a chemical formula of
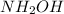
This compound is formed by the combination of nitrogen, hydrogen and oxygen atoms.
In 1 mole of hydroxylamine, 1 mole of nitrogen atom, 3 moles of hydrogen atom and 1 mole of oxygen atom is present.
So, in 2 moles of hydroxylamine,
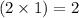 moles of nitrogen atom,
moles of nitrogen atom,
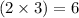 moles of hydrogen atom and
moles of hydrogen atom and
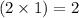 moles of oxygen atom is present.
moles of oxygen atom is present.
Hence, there are 6 hydrogen atoms present in 2 moles of hydroxylamine.