Answer: The correct answer is Option D.
Step-by-step explanation:
For the given chemical reaction:
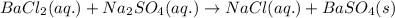
The above chemical reaction is considered as double displacement reaction because here exchange of ions takes place.
In these type of reactions, oxidation state of the atoms remains the same.
On the reactant side:
Oxidation state of barium = +2
Oxidation state of chlorine = -1
Oxidation state of sodium = +1
On the product side:
Oxidation state of barium = +2
Oxidation state of chlorine = -1
Oxidation state of sodium = +1
Thus, the correct answer is Option D.