Answer:
There are 0.5 moles in 11.2 liters of methane at standard conditions.
Explanation:
Standard state conditions are defined by Standard Temperature & Pressure (STP) with a temperature of 0 °C or 273.15 Kelvin (K) and a pressure of 1 atmosphere (1 atm = 101 325 Pa), temperature.
At STP 1 mol of ideal gas occupies 22.4 L.
We know the volume of methane V = 11.2 liters and 1 mol of ideal gas occupies 22.4 L.
To find the moles of the gas we can apply the following formula:
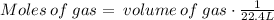
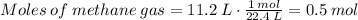
There are 0.5 moles in 11.2 liters of methane at standard conditions.