The ratio of
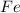
to
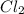
can be determined via the stoichiometric ratios of the reactants in the given equation:
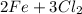
→
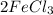
where the numbers in front of the elements/molecules are the stoichiometric ratios. Since we are concerned with the two reactants, we can say that
the ratio of
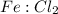 is equal to
is equal to
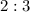
.