Answer:
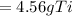
Step-by-step explanation:
Hello,
In this case, by knowing that the Avogadro's number is helpful to relate the atoms with moles and subsequently the atomic mass to relate mole with mass, it is possible to compute the grams of titanium by using its atomic mass as follows:
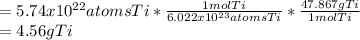
Best regards.