In the equation:
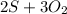
→
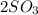
we have 2 moles of
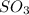
. This means that we have 2 moles of sulfur atoms. So in order to find how many atoms we have, we need to multiply by Avogadro's number. Let's set that up and solve for atoms:
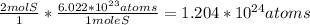
So by using the conversion with Avogadro's number, we are able to determine the number of atoms from the amount of moles we are given.
The amount of sulfur atoms in the products of the reaction are
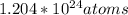
.