Answer: D. like dissolves like
Step-by-step explanation:
The solubility of substances is governed by: Like dissolves like, which states that polar compounds are soluble in polar solvents and non polar compounds are soluble in non polar solvents.
Hydrocarbons are non polar in nature due to less difference between the electronegativities of carbon and hydrogen and thus are soluble in non polar solvents only.
Ionic compounds which are formed by elements with high electronegativity difference are polar in nature and thus dissolve in polar solvents.
Example:
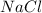 in water.
in water.