The final temperature : 317 °C
Further explanation
Given
V₁=2.5 L
V₂ = 5 L
T₁ = 22 + 273 = 295 K
Required
The temperature, T₂
Solution
Charles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature
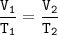
Input the value :
T₂ = (T₁ x V₂) : V₁
T₂ = (295 x 5) : 2.5
T₂ = 590 K = 317 °C