The chemical equation for the reaction of decomposition of 2 moles of Nickel(III) oxide forming nickel and oxygen gas is written as:
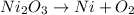
In order to balance the equation we will multiply
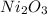 with 2 on reactant side and
with 2 on reactant side and
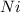 with 4 and
with 4 and
 with 3 on the product side. Thus, the balanced chemical equation is:
with 3 on the product side. Thus, the balanced chemical equation is:
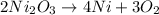
From the balanced equation, it is clear that 2 moles of
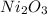 decomposes to give 4 moles of
decomposes to give 4 moles of
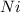 and 3 moles of
and 3 moles of
 .
.
Hence, it is clear from the balanced equation that 4 moles of Nickel are there for every 3 moles of oxygen gas.