First, find the equation for
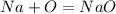
Oxygen hangs out in groups of 2 when alone so you have
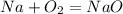
Next, to balance NaO you see that Na has a charge of +1 and O has -2
You need 2 Na for the one O and now you have
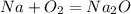
Now you need to balance. 2 Os are on the left so make
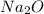
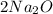
Now you have 4 Na on the right, just make Na, 4Na
Now you have the equation
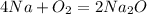
Transfer grams to moles:
Divide the given grams (25.6) by the atomic weight of our variable (Na = 22.99)
25.6/22.99= 1.11352762 This is the number of moles you have.
Use the ratio of the amount of the element or compound to the one you want
It'll look like this
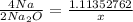
Multiply 1.11352762 by 2 then divide by 4 leaving you with
0.55676381
Finally, multiply that by the atomic mass of
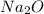
Na2 = 22.99*2 = 45.98+ O = 16 =
61.98g of
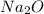 is your answer
is your answer