Answer: Option (D) is the correct answer.
Step-by-step explanation:
A reducing agent is defined as an element or compound which accepts electron(s) and its oxidation number reduces.
For example,
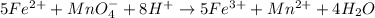
Here, oxidation state of
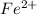 changes to
changes to
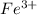 , that is, it is oxidizing.
, that is, it is oxidizing.
Whereas oxidation state of
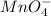 changes from
changes from
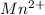 , that is, changes from +7 to +2.
, that is, changes from +7 to +2.
This means
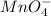 is the reducing agent.
is the reducing agent.