Correct Answer: Mass of ore that contains 200 g of Ti is
617.28 g.
Reason:
Given: Ore contains 32.4% Ti by mass.
It implies that, 100 g of ore ≡ 32.4 g of Ti
Therefore, x g of ore ≡ 200 g of Ti
Thus, x =
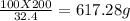
Hence, mass of ore that contains 200 g of Ti is
617.28 g.