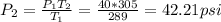In this case, volume of the can remains constant. The relationship between pressure and temperature at constant volume is given by:
P/T = Constant
Then
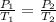
Where
P1 = 40 psi
P2 = ?
T1 = 60°F ≈ 289 K
T2 = 90°F ≈ 305 K (note, 363 K is not right)
Substituting;