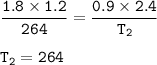Temperature does not change, remains 264 K
Further explanation
Given
V₁= 1.2 L
P₁ = 1.8 atm
T₁ = 264
Required
The new temperature
Solution
The volume was doubled = 2V₁ = 2 x 1.2 = 2.4 L(V2)
The pressure was halved = 0.5P₁ = 0.5 x 1.8 = 0.9 atm(P2)
Combined gas law :
P₁V₁/T₁=P₂V₂/T₂
Input the value :