We know that propane is represented by the chemical formula:
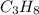
So the molar ratio of carbon to hydrogen is 3:8.
If we are given 0.2 moles of carbon, we can setup a proportion and solve for the number of moles of hydrogen present:
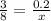
Cross multiply and solve for x (moles of H):
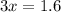
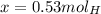
So now we know that when we are given 0.2 moles of carbon in propane, we get a respective 0.53 moles of hydrogen that are present.