The number of neutrons present : 11
Further explanation
Given
²¹Ne
Required
Number of neutrons
Solution
In the following element notation,
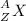
X = symbol of element
A = mass number
= number of protons + number of neutrons
Z = atomic number
= number of protons = number of electrons, on neutral elements
So the symbol above shows the mass number Ne: 21
Ne itself has an atomic number of 10, so the number of neutrons :
number of neutrons = mass number - atomic number
number of neutrons = 21 - 10
number of neutrons = 11