The unknown substance : Lead
Further explanation
Given
Density of element
mass of unknown substance = 40 g
volume = 3.5 cm³
Required
The density of unknown substance
Solution
Density is the ratio of mass per unit volume
The unit of density can be expressed in g/cm³ or kg/m³
Density formula:
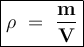
Input the value to find the density of an unknown substance :
ρ = 40 g : 3.5 cm³
ρ = 11.43 g/cm³
If we look at the table, the substance which has a density of 11.43 is Lead