Answer: The molecules are
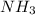 and
and
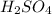 .
.
Step-by-step explanation:
Hydrogen bonding is defined as weak electrostatic force present between the hydrogen atom on one molecule with neighboring electro-negative atom of an other molecule. Usually oxygen nitrogen and fluorine atoms forms hydrogen bonding when present in a molecule of a compound.
From the given compounds , compound with any atom out of oxygen nitrogen and fluorine will form hydrogen bonding with the hydrogen atom present in a molecule adjacent to them.
The molecules are
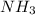 and
and
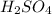 .
.