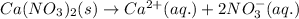Answer: The correct answer is
Step-by-step explanation:
A strong electrolyte is defined as the electrolyte which completely dissociates into its ions when dissolved in water.
A weak electrolyte is defined as the electrolyte which does not completely dissociates into its ions when dissolved in water.
A solution having more number of ions will easily conduct electricity.
For the given chemical equations:
1.
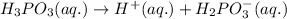
It is a weak electrolyte because it is not completely dissociated and number of ions present are 2.
2.
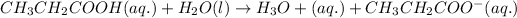
It is a weak electrolyte because it is not completely dissociated and number of ions present are 2.
3.
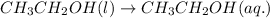
Here, hydration of ethanol is taking place and no ions are present in the solution.
4.
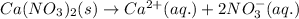
It is a strong electrolyte because it is has completely dissociated into its ions and number of ions present are 3 and thus it will conduct electricity.
Therefore, the solution which will conduct electricity extremely well will be