Answer: The given reaction is a type of substitution reaction.
Step-by-step explanation:
Addition reaction is defined as the chemical reaction in which more atoms are added to the given compound. No atom is lost during this particular reaction. In organic chemistry, unsaturated hydrocarbons (alkenes and alkynes) shows thsi type of reaction.
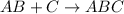
Substitution reaction is defined as the chemical reaction in which one atom replaces the other atom from a compound. In organic chemistry, this reaction is favoured by saturated hydrocarbons (alkanes) only.
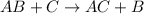
Hydrogenation reaction is defined as the chemical reaction in which hydrogen atoms are increased in a given compound. This is more favoured by alkenes and alkynes (unsaturated hydrocarbons).
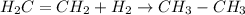
The given chemical reaction follows:
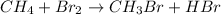
In the above reaction, bromine is substituting hydrogen from methane molecule to form methane bromide. So, the above reaction is a substitution reaction.