Answer:
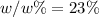
Step-by-step explanation:
Hello,
In this case, since potassium sulfite is in an aqueous solution, that is water as solvent, one could assume 0.0328 moles of potassium sulfite and 1 mole of solution (total), therefore, the moles of water are:
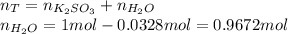
Thus, one calculates each compound's grams by using their molar masses as:
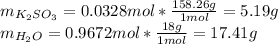
Therefore, its weight/weight % turns out:
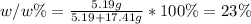
Best regards.