Answer: 0.10 M HCl, pH = 1
Explanation: pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration and pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2019/formulas/physics/high-school/iidawris7irvi0bu33z0a9xjzagtaknk6o.png)
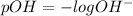
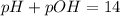
1. 0.10 M
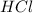 , pH=1
, pH=1
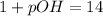
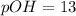
2. 0.001 M
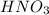 , pH=3
, pH=3
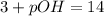
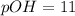
3. 0.01 M
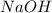 , pH=12
, pH=12
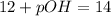
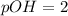
Thus 0.10 M HCl, pH = 1 has highest pOH.