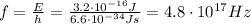A quantum of electromagnetic radiation is called photon, and the energy of a photon is given by
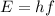
(1)
where h is the Planck constant and f is the frequency of the photon.
In this problem, the photon energy is
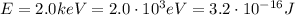
If we re-arrange equation (1), we can find the energy of the photon: