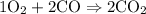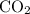
has 2 oxygens for every carbon.
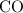
will contribute one of each for every molecule. You then have to have an even multiple of
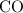
molecules to not end up with an odd number of oxygens.
So if you take
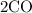
, you should end up with 2 carbons on the RHS.
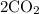
then has 4 oxygens. 2 of them are already accounted for, so you just need one
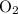
to balance out the reaction.