Answer : The solubility of gas at pressure 250 kPa is, 1.39 g/L
Step-by-step explanation:
According top the Henry's Law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
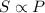
or,

where,
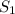 = initial solubility of gas = 0.58 g/L
= initial solubility of gas = 0.58 g/L
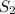 = final solubility of gas = ?
= final solubility of gas = ?
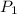 = initial pressure of gas = 104 kPa
= initial pressure of gas = 104 kPa
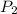 = final pressure of gas = 250 kPa
= final pressure of gas = 250 kPa
Now put all the given values in the above formula, we get the final solubility of the gas.
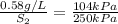
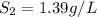
Therefore, the solubility of gas at pressure 250 kPa is, 1.39 g/L