Answer:200/3 M which is approximately equal to 66.6667 M
Step-by-step explanation:Molarity is defined as the number of moles of solute per liter of solution.
It can be calculated as follows:
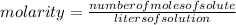
We are given that:
number of moles of solute = 8 moles
volume of solution = 120 ml = 0.12 liters
Substitute with the givens in the above equation to get the molarity as follows:
molarity =
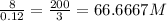
Hope this helps :)