Answer: Option (d) is the correct answer.
Step-by-step explanation:
Transition metals are the metals that contain incompletely filled d-shell. These metals are also known as 3d-series metals and elements of these series show variable oxidation state.
So, while naming these compounds a roman numeral is placed next to the symbol of cation as they show different oxidation states with different anions.
For example, name of compound CuCl is copper(I) chloride. And, name of compound
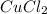 is copper(II) chloride.
is copper(II) chloride.
Hence, we can conclude that a roman numeral is most likely needed in the name of an ionic compound when the cation is a transition metal.