Answer: 8:1
Step-by-step explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The give balanced chemical equation is:
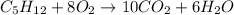
According to stoichiometry:
8 moles of oxygen reacts with 1 mole of pentane and thus mole ratio of oxygen to pentane is 8:1