Answer: The number of hydrogen atoms that can combine to oxygen are 2.
Step-by-step explanation:
Oxygen is the 8th element of the periodic table with electronic configuration:
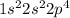
This element requires 2 electrons to attain stable electronic configuration.
Hydrogen is the 1st element of the periodic table with electronic configuration:
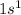
This element will loose 1 electron to attain stable electronic configuration.
2 Hydrogen atoms will share their 1 electron each with 1 oxygen atom. So, that both the element attain stable electronic configuration.
They will form a covalent compound with chemical formula

Hence, the number of hydrogen atoms that can combine to oxygen are 2.