Avagadros law states that volume of gas is directly proportional to number of moles of gas at constant pressure and temperature.
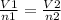
where V -volume , n - number of moles
parameters for the first instance are on the left side and parameters for the second instance are on the right side of the equation
substituting these values in the equation
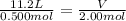
V = 44.8 L
answer is C. 44.8 L