Answer: The silver half reaction occurs at the cathode.
Explanation: In a cell, there exists two electrodes: anode and cathode.
Anode is an electrode at which oxidation reaction occurs which meas that loss of electrons occur at this electrode.
Cathode is the electrode at which reduction reaction occurs which means that the gain of electrons occur at this electrode.
In the give electrochemical cell, the silver half reaction follows:
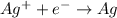
This is a type of reduction reaction because
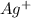 is gaining one electron to form Ag.
is gaining one electron to form Ag.
Hence, it will takes place at the cathode.