Answer:
na2co3 is a stronger base
na2co3 puts more oh- ion into solution
Step-by-step explanation:
Step 1: Calculate the pH of 0.1 M Na2CO3
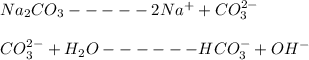
Kb value for Na2CO3 = 2.1 * 10⁻⁴
Set up an ICE table
CO_{3}^{2-} + H_{2} O ------HCO_{3}^{-} + OH^{-} \\[/tex]
I 0.1 - - -
C -x +x +x
E (0.1-x) x x
![Kb = ([HCO3-][OH-])/([CO3^(2-]) ) \\\\Kb = (x^(2) )/((0.1-x)) \\\\2.1*10^(-4) = (x^(2) )/((0.1-x)) \\\\x = [OH-] = 0.00458 M\\\\pOH = -log[OH-] = -log(0.00458) = 2.33\\\\pH = 14 - 2.33 = 11.66](https://img.qammunity.org/2019/formulas/chemistry/high-school/otq63siddnoenfvt5nyy34b1ptiweopkyx.png)
Step 2: Calculate pH of 0.1M NaHCO3
NaHCO3 is amphiprotic i,e it acts as an acid and base

For amphiprotic systems:
![[H+] = √(Ka1 * Ka2) \\\\For H2CO3:\\Ka1 = 4.5 * 10^(-7) \\Ka2 = 4.8 * 10^(-11) \\\\[H+] = √((4.5*10-7 * 4.8*10-11) = 4.65*10-9\\pH = -log[H+] = 8.33](https://img.qammunity.org/2019/formulas/chemistry/high-school/zf5vljvmob2am3o8upfkai3n14snh3rrng.png)
Conclusion:
pH of 0.1 M Na2CO3 = 11.66
pH of 0.1 M NaHCO3 = 8.33
Higher the pH , more basic the solution. Hence, Na2CO3 is a stronger base than NaHCO3