The heat released by the water when it cools down by a temperature difference

is
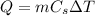
where
m=432 g is the mass of the water
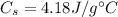
is the specific heat capacity of water
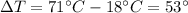
is the decrease of temperature of the water
Plugging the numbers into the equation, we find
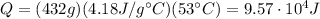
and this is the amount of heat released by the water.