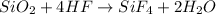Let's start from the Fluorine (F), which has 4 atoms on the right side: this means we must put a factor 4 in front of HF to balance the number of atoms of Fluorine.
Nowe we have 4 atoms of hydrogen, H, on the left side. Since we have a

on the right side (2 atoms), we must put a factor 2 in front of
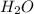
, to balance the number of atoms of hydrogen.
So now the atoms of oxygen are also balanced (2 on both sides), as well as the atoms of Silicon (Si), so the balanced reaction is