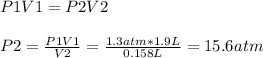Answer:
The final pressure of the gas is 15.6 atm (≅ 16 atm)
Step-by-step explanation:
Given:
Initial volume of the gas, V1 = 1.9 L
Initial pressure, P1 = 1.3 atm
Final volume of the gas, V2 = 158 ml = 0.158 L
To determine:
Final pressure, P2
Step-by-step explanation:
Based on the ideal gas equation
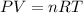
where P = pressure, V = volume ; n = moles of gas
R = gas constant, T = temperature
At constant n and T, the above equation becomes:
PV = constant
This is the Boyle's law
Therefore: