We know that to relate solutions of with the factors of molarity and volume, we can use the equation:
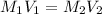
**
NOTE: The volume as indicated in this question is defined in L, not mL, so that conversion must be made. However it is 1000 mL = 1 L.
So now we can assign values to these variables. Let us say that the 18 M
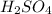
is the left side of the equation. Then we have:
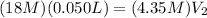
We can then solve for
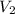
:
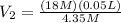
and
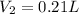
or
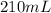
We now know that the total amount of volume of the 4.35 M solution will be
210 mL. This is assuming that the entirety of the 50 mL of 18 M is used and the rest (160 mL) of water is then added.