Answer: For the given electronic configuration, E will be 2, F will be 2 and G will be 1.
Step-by-step explanation:
Boron is the 5th element of the periodic table. The number of electrons present in this element will be equal to the atomic number which is 5.
Electronic configuration of an element is the denoted as the total number of electrons present in that element.
First 2 electrons will be filled in 1s orbital. Then two electrons will be filled in 2s-orbital and rest in 2p-orbital.
Hence, the electronic configuration of Boron becomes:
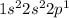
Therefore, E will be 2, F will be 2 and G will be 1.