Answer : Sulfuric acid is a strong acid.
Explanation :
Acids are of 2 types
1) Strong acid : This is the type of acid that completely dissociates forming H⁺ and an anion.
A strong acid has a very large Ka value.
2) Weak acid : Weak acid have a tendency to dissociate partially. They do not release all the H⁺ ions. Only a fraction of the molecules can release the H⁺
Weak acids have small Ka values.
The chemical formula of sulfuric acid is H₂SO₄. It dissociates as follows.
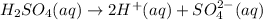
Ka value for H₂SO₄ is approximately 1 x 10³.
Therefore we can say that H₂SO₄ is a strong acid.