Answer: The pH of the solution is 5 and the solution is acidic in nature.
Step-by-step explanation:
pH is defined as the concentration of hydrogen ions in a solution. It is basically the negative logarithm of hydrogen ion concentration. It is known as power of hydrogen.
Mathematically,
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
It is a scale ranging from 0 to 14. If the pH of the solution ranges from 0 to 6.9, the solution is said to be acidic and if the pH ranges from 7.1 to 14, the solution is said to be basic. If the pH of the solution is 7, the solution is a neutral solution.
We are given:
![[H^+]=1* 10^(-5)](https://img.qammunity.org/2019/formulas/chemistry/high-school/f9owco1p9vxs48jfx4mu6pulbprdtj7ri5.png)
Putting this value in above equation, we get:
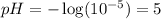
As, the pH of the solution comes out to be 5, the solution will be acidic in nature.